THE BUSINESS
In 2015, life science industry veteran Connie Lebakken, PhD, teamed with UW-Madison Professor Bill Murphy, PhD to launch Madison-based biotech startup Stem Pharm. The business was founded around two key technologies – novel nature-inspired biomaterials and 3D brain organoids, enabled by these biomaterials, discovered by Dr. Murphy and collaborators at UW. The company’s leadership team has since grown to include Steven Visuri, PhD, CEO, and Ryan Gordon, PhD, Sr. V.P. of Business Development, who together bring decades of pharma-relevant business and operational experiences to the company.
Neurological diseases are unimaginably complex, making neurological drug discovery extremely challenging. FDA approval rates for new drugs are nearly half that of non-neurological drugs – for neurodegenerative diseases, the failure rate is nearly 100%. Rodent models, commonly used in drug discovery and preclinical development, are poor predictors of neurological diseases. One approach to improve the quality of the neurological drug candidates is to create biologically relevant human cell-based models of these diseases and implement them into early drug discovery, prior to testing in animals, when new targets are identified and lead compounds are selected.
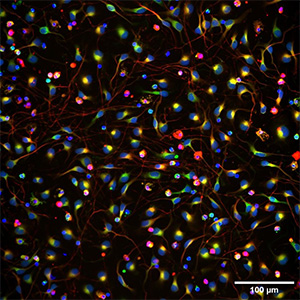
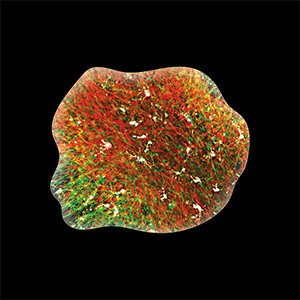
Based on its founding technologies, Stem Pharm has developed a proprietary drug discovery platform based on human stem cell derived 3D neural organoids (“brains-in-a-dish”) that uniquely incorporate the brain’s resident immune cells called microglia. Inspired by biology, Stem Pharm simulates the natural environment so the brain cells self-assemble as they would in development. The resulting organoids reflect the complexity of cell types and important cell-to-cell interactions in the developing brain and can be used to model neurological diseases.
Because they contain microglia, Stem Pharm’s neural organoids are particularly suited to model human neuroinflammation, which plays a critical role in many neurological diseases and disorders. They have demonstrated that the organoids can generate neuroprotective and neuroinflammatory responses that are critical in disease formation or progression. By combining the ability to model complex human neural biology with a rich set of analytical tools, Stem Pharm utilizes the organoids for the discovery of new therapeutic approaches for neurodegenerative diseases, brain tumors, and epilepsy.
Stem Pharm’s innovation enables the company, and its biopharma partners, to explore new disease pathways and mechanisms, identify and validate drug targets, and test the neuroinflammatory effects of therapeutics in a highly reproducible human model. The company was recently awarded two grants from the National Institutes of Health (NIH) to utilize its organoid platform to develop disease models for drug discovery, including a Small Business Innovation Research (SBIR) grant to develop a human model of Alzheimer’s Disease and a Small Business Technology Transfer (STTR) grant to develop a human model of glioblastoma brain cancer. Stem Pharm also recently announced a collaboration with Verge Genomics, an AI-driven biotech company, to develop a Parkinson’s Disease model to validate novel targets identified by Verge.
THE RESOURCES
Stem Pharm has received multiple levels of support from the Center for Technology Commercialization (CTC), including support in drafting SBIR grant applications, matching grants through CTC’s SBIR Advance program, and fundraising and commercialization mentoring. Steven said, “Bill Murphy’s lab at the UW-Madison developed the foundational technology in 2015. Since that time, Stem Pharm has made improvements to the technology and validated its use using grant funds for advancing the technology and reducing technical risks. The feedback provided from CTC has strengthened our SBIR applications and helped us successfully obtain multiple NIH grants.” In addition, Stem Pharm has been awarded SBIR Advance grants from the CTC that funded non-technical activities such as commercialization activities and preparing for fundraising.
“The assistance we received from CTC was fantastic! The SBIR Advance funding has been instrumental in our success. Early on, valuable coaching was provided by CTC on learn startup concepts. We also learned a lot from working alongside other startup companies being mentored by CTC,” Steven said.
“We gained valuable insights working with CTC. They were incredible mentors and provided wonderful feedback on our business model, marketing materials and pitch deck presentation. While our company leaders have significant biotech industry experience, the CTC mentors provided different perspectives based on their vast experiences,” Steven said.
The team was recently awarded a Small Business Technology Transfer (STTR) grant from the National Cancer Institute (NCI) to advance cutting-edge research in a form of brain cancer. The grant will support the development of an innovative neural organoid model to study the immunologic microenvironment of glioblastoma, ultimately paving the way for novel drug discovery applications. Stem Pharm is collaborating on this grant with researchers at UW-Madison, Dr. Mahua Dey and Dr. Christian Capitini.
Stem Pharm’s work is important to the many biotech startups in the local community. “Madison is a hub for stem cell innovation and being here allows us to partner in important life science collaborations,” Ryan said. In addition to their glioblastoma collaboration, they work with other researchers and professors at the UW-Madison and hosted a PhD student intern from UW-Madison to solve data science challenges for the company. They also collaborate with several biotech companies in the Madison area.
THE FUTURE
Steven said, “What is most important to us is building an effective technology that will impact patients’ lives. For us, this means creating better drugs for treating neurological disease.”
The future holds many goals for the team. “In the near-term, we will validate disease models for Alzheimer’s, Parkinson’s and epilepsy,” Steve said. They are also working to raise the next round of funding for the drug discovery phase.

